Merck's Path To Continuous Manufacturing For Solid Oral Dose Products: What Stands In The Way?

By Trisha Gladd, Editor, Life Science Connect

As the evolution of the global pharmaceutical industry continues to drive the need for more flexibility and lower costs, continuous manufacturing becomes an even more appealing and sensible option. However, despite the tremendous promise of its economic and quality control benefits, there are still many concerns about the regulatory landscape for continuous manufacturing. Dr. Christine Moore, executive director and global head of chemistry, manufacturing and control (CMC) policy at Merck, recently discussed the company’s pursuit of worldwide approval of continuous manufacturing for solid oral dosage products and what regulatory risks it sees as potential roadblocks.
Life Science Leader (LSL): What does Merck see as the major benefits of continuous manufacturing?
Moore: There are three main advantages we see for continuous manufacturing: agility, supply predictability, and quality assurance. From an agility perspective, continuous manufacturing offers the potential to have flexible batch sizes, which will allow Merck to quickly respond to changes in patient demand. From a predictability of supply perspective, continuous manufacturing offers an ability to improve overall reliability and robustness, leading to decreased potential for drug shortages.
With regard to quality assurance, continuous manufacturing systems are designed with integrated measurements and controls that allow for real-time analysis and control. Because of the high level of measurement and control, these systems have the ability to detect upsets in the process and to segregate potentially non-conforming material from the rest of the product. Additionally, the continuous blenders have intimate contact between the materials, so there can be a decrease in variability due to the more uniform shear than from a large batch blender. Early evidence from academic researchers shows that material from continuous manufacturing equipment is less likely to segregate. It’s also probable—although I have not seen data yet—that continuous manufacturing could decrease dissolution variability for shear-sensitive products because of more uniform exposure to shear for the lubricants.
LSL: Why does Merck see continuous manufacturing as a promising way to successfully expand its global footprint for solid oral dose products?
Moore: What we utilize for manufacturing traditionally and what is currently used today are large, centralized facilities making very large batches. Inventory levels tend to fluctuate, depending on where you are in your production cycle. The direction we are moving is toward smaller-volume products as well as the desire to have localized manufacturing closer to the customer.
There is an ongoing effort driven by Merck Manufacturing Division called “World Class Supply.” The goal is to have a total lead time of 90 days from formulation to the patient, which is about one-quarter of the current time. These shorter timelines and lower inventories will necessitate that we quickly respond to changes in the market. We want to be manufacturing material flexibly from week to week, driven by how much we sold the week before. In order to do this, we need to have well-controlled manufacturing processes and flexible batch sizes. Merck sees continuous manufacturing as a way to achieve these goals.
LSL: How does Merck plan to accomplish this?
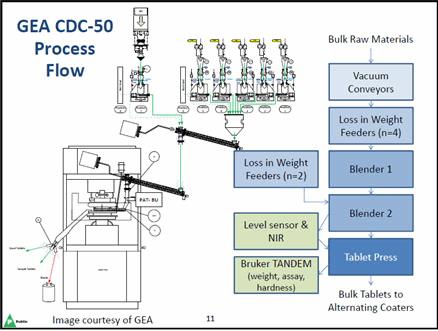
Dr. Moore: In order to take a step toward our goal of World Class Supply, Merck is moving forward with a proof of concept operation using an example product that is easy to manufacture and has a very well-controlled process. We are going to build upon our existing quality by design (QbD) and real-time release testing (RTRT) approaches already approved for this product. We will operate with short duration runs and flexible batch sizes, including efficient changeovers between strengths. The continuous manufacturing equipment is quite simple, but advanced automation is needed for the whole system to run in an integrated fashion. We are using a direct compression process where initially raw materials or API are transferred through vacuum conveyors to Loss in Weight (LIW) feeders. Four feeders precisely transfer the three different excipients and the API to the first continuous blender. Lubricants are then added and the material is put through another blender, followed by the tablet press. Process analytical technology (PAT) is applied via sensors after the second blender and the speed of the tablet press is adjusted to match the rate of the compression to that of the blending. Additionally, PAT measurements and/or process models are utilized to determine the quality of the product during the run; any potentially non-conforming material is segregated from the product. After tablet compression, a Bruker TANDEM (a fully automatic, online PAT tool that collects process data) is used to simultaneously measure tablet attributes, such as weight, assay, and hardness. Tablet disintegration or process models are used in lieu of traditional dissolution testing. Finally, the tablets go to two alternating coaters. While one coater is running, the second coater is filling up, and then that coater runs while the first coater fills up, to provide continual output of product.
While many companies are developing continuous manufacturing processes for solid oral dosage forms, I personally don’t know if any other company is planning to file worldwide. We plan on filing this approach as an additional manufacturing process scheme to our currently approved batch process rather than a full changeover of our manufacturing from batch to continuous. The experience that we gain, both from a technology and a regulatory perspective, will help us chart our path forward on continuous manufacturing, and it may help move the pharmaceutical industry forward as a whole.
LSL: What progress have you seen from regulators when it comes to continuous manufacturing?
Dr. Moore: In April 2016, Dr. Lawrence Yu, deputy director of the FDA’s Office of Pharmaceutical Quality, highlighted in an FDA blog two significant approvals of applications containing continuous manufacturing technology. The first was the use of continuous manufacturing by Vertex to produce its cystic fibrosis drug, Orkambi. The second was the first approval by the FDA of a manufacturer’s change in their production method from batch to continuous for Janssen’s drug Prezista for the treatment of HIV-1 infection. These are both positive steps moving the technology forward.
Merck has seen great support from regulators in the major regions, including US, EU and Japan. There have been positive interactions and a strong willingness to meet with companies about continuous manufacturing technology. These agencies all have specialized teams to support new technologies, including the FDA’s Emerging Technology Team, the EMA’s PAT Team and the Innovative Manufacturing Technology Work Group, which is newly established by the Pharmaceutical and Medical Devices Agency (PMDA) in Japan. There have also been several collaborations between the industry and regulators, including the EMA/FDA QbD pilot, which included applications for continuous manufacturing. Additionally, several regulatory and technology white papers have either been published or are in progress, many in collaboration from regulators. These include efforts from the Massachusetts Institute of Technology (MIT), the Engineering Research Center for Structured Organic Particulate Systems (C-SOPS), ISPE, and USP. We are also seeing a growing number of conferences outside of the US, including in China and Japan. However, there are little or no conferences in other emerging regulatory regions, such as Southeast Asia or Latin America. Outreach and education in these emerging regions will be important for Merck as well as other pharmaceutical companies that are looking to expand this technology.
LSL: What regulatory concerns do you have about continuous manufacturing?
Dr. Moore: Based on the progress we are seeing with the technology and the willingness of regulators to move continuous manufacturing forward, the overall landscape is positive. There are some areas, though, where we have concerns and that could be potential roadblocks for adding continuous manufacturing to our current applications for legacy products.
One lesser concern is the ability to add continuous manufacturing to a batch manufacturing in the same application to allow for the greatest flexibility. We believe that this approach is consistent with ICH [International Conference on Harmonisation] guidelines that describe different control strategies for the same product. However, not all regions adhere to ICH. We also have little concern about the definition of “batch” for continuous manufacturing. In general, industry and regulators are in full agreement that a batch can be defined based upon an amount of material or a length of time, and that traceability is important.
One question we are seeking feedback on from different health authorities is whether it is okay to tweak the formulation when switching to continuous, such as to increase or decrease the amount of a lubricant. We anticipate that we might not be able to eliminate or replace an excipient because we want the continuous and batch product to be interchangeable with the same inactive ingredients on the label. Flexible batch size is another area where there is some uncertainty. It is an important component needed to achieve Merck’s World Class Supply goals.
We think the most important aspect is to have common control strategies approved worldwide. Merck uses a term called product “flavors,” which are products that can only be marketed in limited regions, based upon approved dossier requirements. Flavors can arise from different control strategies at the time of approval or different timelines for post-approval changes, such that some markets can only get pre-change material and some can get post-change material. This situation can lead to a lot of complexity, and increased complexity increases risk.
Additionally, certain process control variants, like different approved spectroscopy models, would be very difficult if not impossible to manage in manufacturing. The costs of regulatory divergence are not only costs to the industry but also costs related to public health issues. This is because of the increased likelihood of stock-outs and shortages and a potential delay of the implementation of innovative technologies. We are actively working to help regulators understand these potential challenges and to strive for alignement on control strategies, which will help move this technology forward both for legacy and new products.
LSL: How does the industry move forward with new technologies without the assurance of global acceptability?
Dr. Moore: Merck is always concerned about the ability of getting our drugs to patients who need them worldwide. There are some aversions to new technology when the global regulatory acceptability is not yet known, which is a major reason why we are starting our continuous manufacturing efforts on an already-approved product. Also, from an equipment perspective, it becomes somewhat of a chicken and egg problem. What comes first? Does the capital investment drive the new technology or do you wait for the new technology to drive the capital investment? Moving forward for any company, there must be proactive leaders who are willing to take the plunge to support and invest in new technology, including continuous manufacturing.
